In my day-to-day work as a GLP consultant, I often find myself reaching for a formal definition of some term from the GLPs. This can be useful when I want to explain a concept, reference the definition, or verify the exact wording. I wanted to be able to go to one place to easily search these definitions. So I built a GLP definitions browser. It’s freely available for anyone to use, and you can find it at:
https://readtheregs.com/glp/define
Here’s a video of it in action:
The GLP Definitions browser has all of the formal definitions from the main OECD, FDA and EPA GLPs. Specifically, this includes the documents OECD Principles of Good Laboratory Practice #1, 21CFR Part 58, and 40CFR Parts 160 and 792).
There is a simple search and filter mechanism so you can quickly find exactly what you’re looking for:
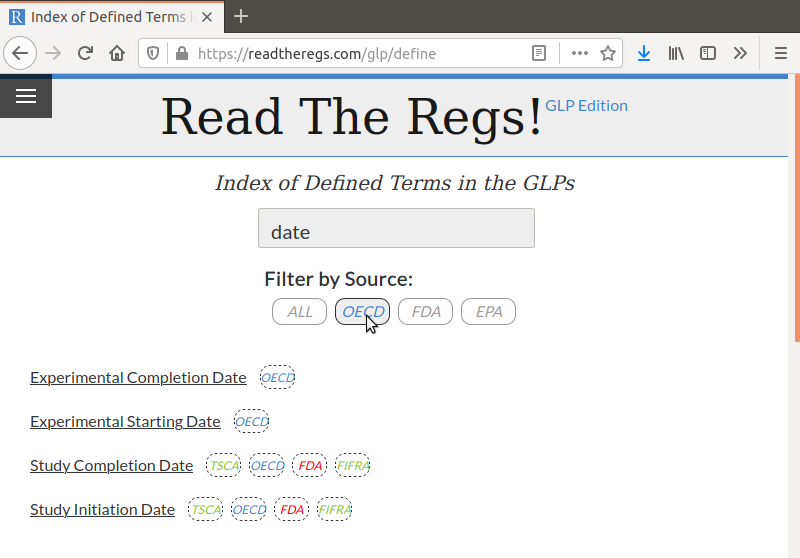
Because there are often differences in terminology between the various GLPs, I built in support for synonyms. For example, clicking on Experimental Start Date will list the EPA definitions as well as the OECD definition for Experimental Starting Date:
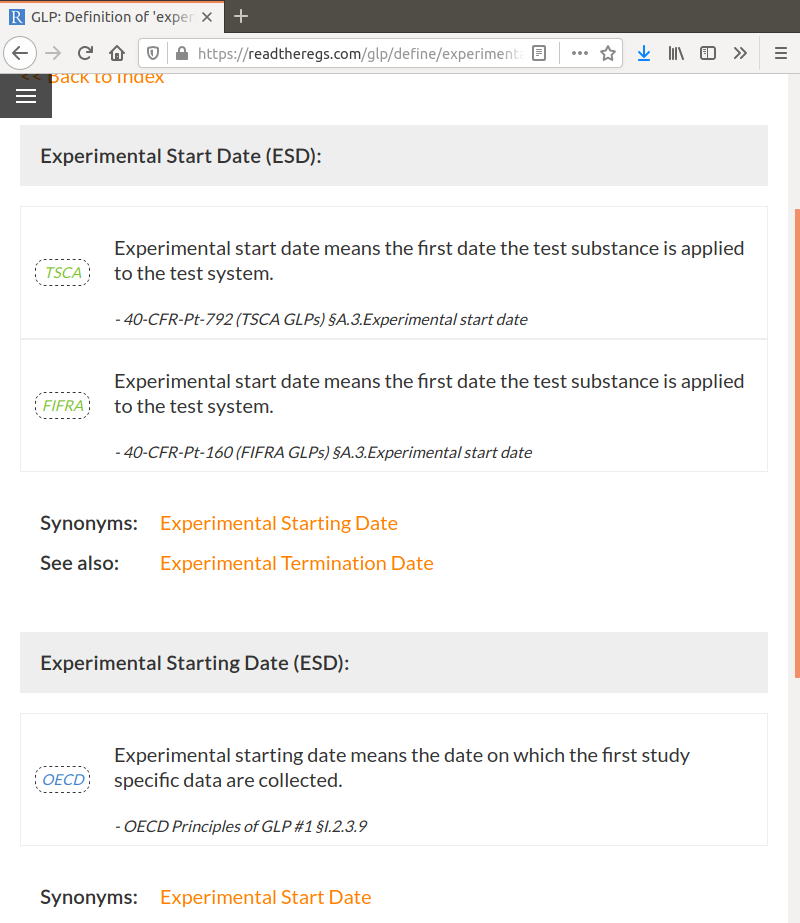
Along with synonyms, links to related terms are also given. For example, you can see above that the Experimental Termination Date is given as a related term for the EPA definitions, while in the OECD definition a link to the Experimental Completion Date is suggested instead.
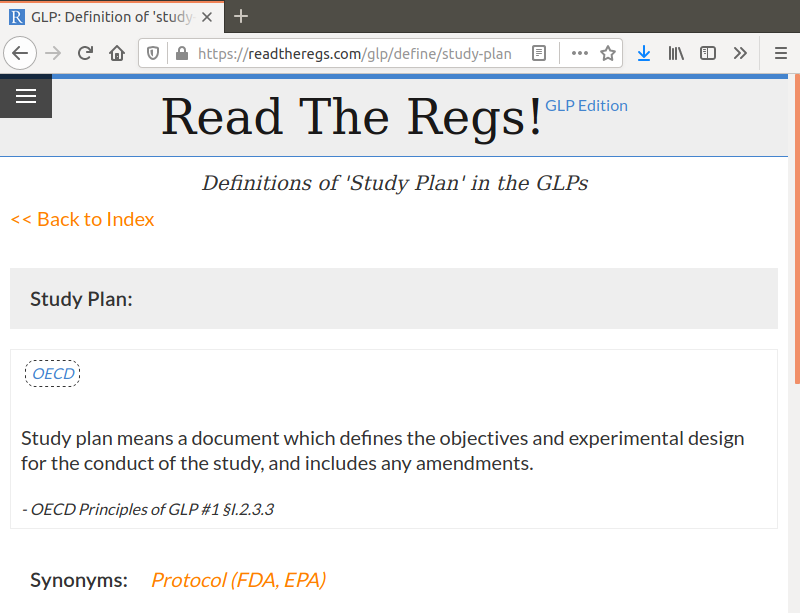
You can even link directly to any given definition by typing in the term at the end of the URL, replacing any spaces with dashes. For example, linking directly to https://readtheregs.com/glp/define/study-plan gives you:
There is more to come, including added definitions from related topics such as Part 11 and the CSV guidance.
So give it a try today and look up a GLP definition.


Hey, Brendon. Any info on Canadian GLP?
Hi Eric, thanks for reaching out!
Yes, Canada follows the OECD Principles of GLP and both the Definitions Browser and ReadTheRegs have the main OECD GLP document (#1 in the series) covered. The Pro version of Read the Regs also has several of the other OECD guidance documents as well, with more to come. I hadn’t considered including the Canadian regulatory directives for GLP as mainly just point to the OECDs. They do provide some specific requirements for records retention, so I may consider that for a future release if there’s demand for it.